Systane brand eye drops were voluntarily recalled because of possible fungal contamination, the Food and Drug Administration said.
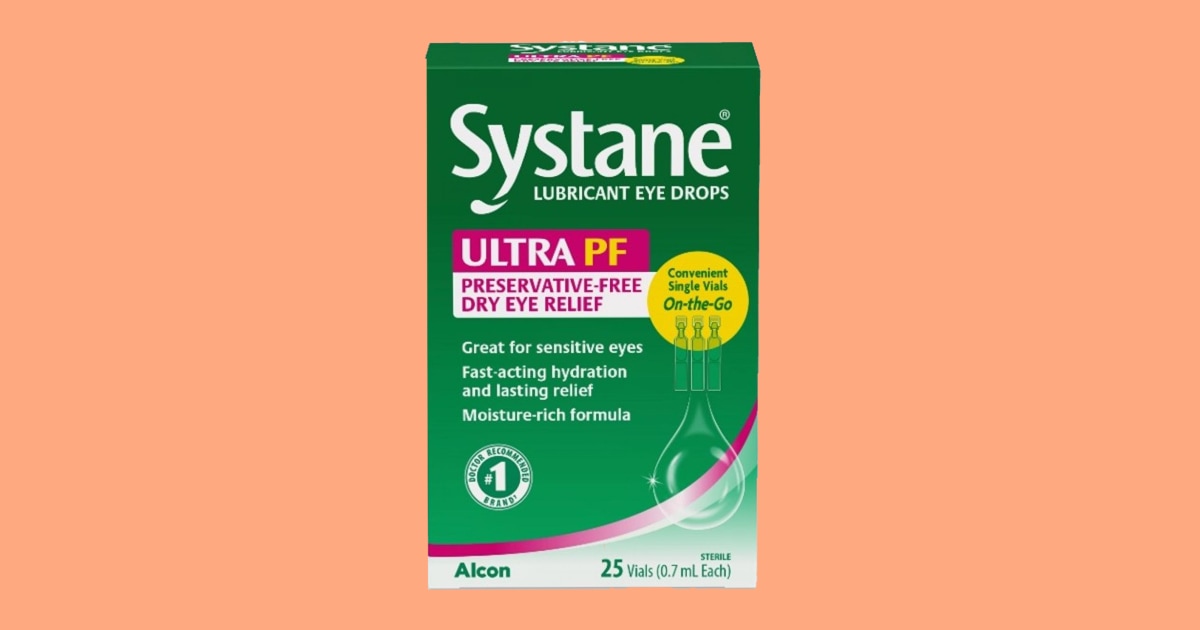
One lot of Systane Lubricant Eye Drops Ultra PF was recalled following a consumer complaint “of foreign material observed inside a sealed single-use vial,” the FDA said in a release. It was determined that the material was “fungal in nature.”
The contamination could potentially cause eye infections that “may be vision-threatening, and in very rare cases potentially life-threatening in immunocompromised patients,” according to the release.
Alcon Laboratories, which manufacturers the eye drops, has not received any reports of customers suffering adverse effects, the FDA said.
The recalled eye drops come in the 25-count on-the-go single vials with lot number 10101 and an expiration date of September 2025. The product was sold at Publix, according to a notice from the grocery chain.
The drops are used to treat burning and irritation in people experiencing dry eye symptoms.
Anyone who has purchased the eye drops should stop using them immediately and can seek either a refund or replacement, the FDA said. If anyone is experiencing symptoms, they should consult with their physician.
Steven Smith, an Alcon spokesperson, said that the company’s investigation is ongoing, but “the presence of foreign material appears to be isolated to the single unit returned by a customer.”
Alcon recalled the product “out of an abundance of caution to prioritize consumer safety,” he added.